The AME2012 Atomic Mass Evaluation
Some content in Image.. Sorry my ImagePathDesign is not ready, but orcad give about perfect conversion and PDF2DJVU seems to work fine with...
Few example about the content :
Units; recalibration of α- and γ-ray energies
Atomic mass determination for a particular nuclide can be generally performed by establishing an energy relation between the mass we want to deduce and that for a well known nuclide. This energy relation is then expressed in electron-volts (eV). Mass values can also be obtained as an inertial mass from the movement characteristics of an ionized atom in an electro-magnetic field. The mass, is
then derived from a ratio of masses and it is then expressed in ‘unified atomic mass’ (u). Those two units are used in the present work.
The mass unit is defined, since 1960, as one twelfth of the mass of one free atom of carbon-12 in its atomic and nuclear ground states, 1 u = M(12 C)/12. Before 1960, two mass units were used: the physics one, defined as of nucleons in a molecule, at least as it is presently used in these domains. It is thus not strictly the same as ‘u’. The unit for energy is the electron-volt. Until the end
of last century, the relative precision of M −A expressed in keV was for several nuclides less accurate than the same quantity expressed in mass units. The choice of the volt for the energy unit (the electronvolt) is not unambiguous.
For example, one may use the international volt V, but other can choose the volt V90 as maintained in national metrology laboratories and defined by adopting an exact value for the constant (2e/h) in the relation between fre-
quency and voltage in the Josephson effect. Since 1990, by definition 2e/h = 483597.9 (exact) GHz/V90 (see Table B). Already in 1983, an analysis by Cohen and Wap-stra [16] showed that all precision measurements of re-action and decay energies were calibrated in such a way that they can be more accurately expressed in maintained volt. Also, as seen in Table A, the precision of the con-
version factor between mass units and maintained volt (V90 ) is more accurate than that between the former and international volt. In fact, the accuracy is so high that the relative precision of M − A expressed in eV90 is the
same as that expressed in mass units. For example, the mass excess of 4 He is 2 603 254.13±0.06 nu in mass units, 2 424 915.63±0.06 eV90 in maintained volt units and 2 424 915.78±0.08 eV in international volt units. Due to the increase of precision, the relative precision of MA expressed in keV90 is as good as the same quantity ex-pressed in mass units, whereas the uncertainties expressed
in international volts are larger than in V90 . Therefore, as already adopted in our previous mass evaluations, the V90 (maintained volt) unit is used in the present work. In the most recent (2012) evaluation by Mohr et al.
[13], the relation between maintained and international volts is given as V90 =[1+6.3(2.2)×10−8 ]V, that could be expressed as a difference of 63(22) ppb.
In Table A the relations between maintained and international volts, and several constants of interest, obtained from the evaluation of Mohr et al. [13] are given. Given also are the ratio of mass units to electronvolts for the two...
Now where is the feed to get data-calibration inside my engine !!!
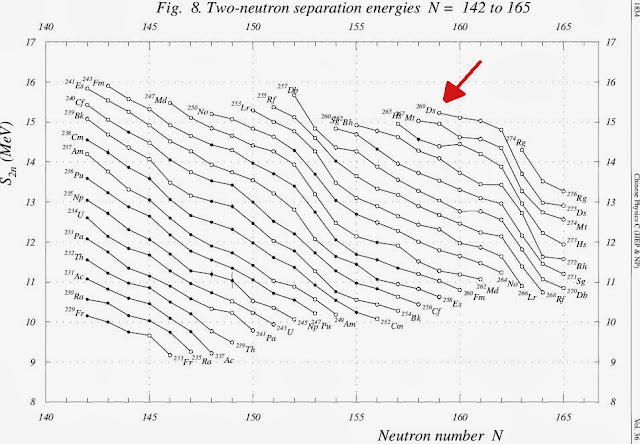
- And what's magic with the Volume II of The AME2012 Atomic mass evaluation, is the page 232 with the Ds element having a graph with Two-neutron separation Energies N=142 to 165, the Ds-269 - Ds-275 had data which is merely impossible with actual technology.. see the graph
| X | Text may contains errors, syntax errors or lexical error... |
| X | Now with SpellChecking and Editable text. Up to you to get something correct. |
1 No Interest 2 Somewhat good 3 Good 4 Special.